Fluorescent protein of freshwater eels used to film a “feature-length film” of the cell.
A team led by Professor Sang-Hee Shim observes cell structure eight times longer than existing technology.

▲ From left, Professor Sang-Hee Shim (corresponding author), Korea University College of Science, Department of Chemistry,
Ji-Woong Kwon (lead author)
A research team led by Professor Sang-Hee Shim (Korea University, Department of Chemistry) of the Institute for Basic Science (IBS, President Do-Young Noh) Center for Molecular Spectroscopy and Dynamics (Director, Korea University Professor Min-Haeng Cho), in collaboration with Seoul National University and Ulsan Institute of Science and Technology (UNIST), developed a super-resolution fluorescent microscopy with the fluorescent protein of freshwater eels that can observe the structure of live cells eight times longer.
* Super-resolution fluorescence microscope: a microscope that overcomes the limitations of optical microscopes that cannot view the microscopic world of viruses and proteins. By controlling the state of the fluorescent protein, live cells can be observed at the molecular level. The three researchers who developed super-resolution fluorescent microscopy were awarded the 2014 Nobel Prize for Chemistry.
The cell, the basic living unit, is a complex system in which various molecules several nanometers in size change dynamically. Super-resolution fluorescence microscopy is required to observe the nanostructures in live cells. However, due to the photobleaching phenomenon, in which the fluorescence disappears when the fluorescent protein is repeatedly exposed to light, it was difficult to film the nanostructures in super-resolution for a prolonged time.
Most fluorescent proteins use the amino acid of the protein itself as an illuminant. Because of this, when exposed to light for a long time, the structure of the protein is damaged, and fluorescence disappears. Using the existing technology, over a prolonged period of time, the intensity of the fluorescent signal gradually decreases and eventually disappears.
The researchers focused on UnaG, a fluorescent protein derived from freshwater eel that uses bilirubin, an external metabolite, instead of an internal amino acid, as a light emitter.
* UnaG: In 2013, researchers from Japan's Rikagaku Kenkyusho (RIKEN) discovered fluorescent proteins in freshwater eel and named them UNaG, reporting them in the international journal Cell. UNaG is the first vertebrate-derived fluorescent protein.
* Bilirubin: Gardenia pigment produced by the breakdown of hemoglobin in red blood cells.
The UnaG protein and bilirubin do not emit fluorescence when they are separate, but when combined, they become a fluorescent material that emits a bright green fluorescence. The researchers found that when blue light is applied to the UnaG-bilirubin conjugate, the fluorescence is turned off by photobleaching and is restored later when treated again with bilirubin. This shows that the blue light and bilirubin solution can be used to turn the fluorescent signal off and on. There is no structural damage to the UnaGi protein after photobleaching. The UnaG protein in a bilirubin aqueous solution repeats separating from damaged bilirubin and binding to fresh bilirubin, which circulates the photobleaching-fluorescence recovery process and can repeatedly produce a fluorescence switching reaction.
The researchers then applied UnaG to a super-resolution fluorescence microscope. After labeling the UnaG on the subcellular structure, blue light was applied to turn off fluorescence. By recombining it with bilirubin, they were able to increase the fluorescence of only a portion of the UnaG. By measuring the location of the molecules in the cell with nanometer accuracy, they were able to construct super-resolution images.
UnaG has the advantage that resolution can be increased by labeling the location of the high density molecules with half the amount of the conventional fluorescent protein. Furthermore, the team succeeded in controlling the reaction rate at which the fluorescence is turned off and recovered through the laser’s intensity and the oxygen concentration in the solution.
This research has developed an observation technique that can continually repeat fluorescence switching at an appropriate rate, making super-resolution video recording that is not limited to photobleaching in live cells possible. Cells can be observed about eight times longer than with conventional technologies, allowing a more accurate picture of the internal structure of cells.
Professor Shim, said, “This technology overcomes the photobleaching limitation that has been a hindrance to filming live cells with ultra-high-resolution fluorescence microscopy. This technology will greatly contribute to the identification of bionanomaterials and biologic phenomena studies that require long-term observation.”
The team's findings were published online in the January 14 issue of the international journal, Nature Communication (IF 11.880).
* Paper Title: “Bright ligand-activatable fluorescent protein for high-quality multicolor live-cell super-resolution microscopy” (Nature Communications)
* Author Information: Ji-Woong Kwon, Jong-Seok Park, Min-Su Kang, Soo-Bin Choi, Ju-Mi Park, Gyeong-Tae Kim, Chang-Wook Lee, Sang-Won Cha, Hyun-Woo Rhee and Sang-Hee Shim

[Figure 1] Structure and switching reactions of UnaG fluorescent proteins
UnaG protein and bilirubin do not fluoresce individually but become fluorescent proteins that emit bright green fluorescence after binding (a). When blue light is applied to an aqueous solution of UnaG fluorescent protein, the fluorescence is turned off. It is then revived when treated with a bilirubin solution. This photobleaching-fluorescence recovery cycle can be repeated many times (b).
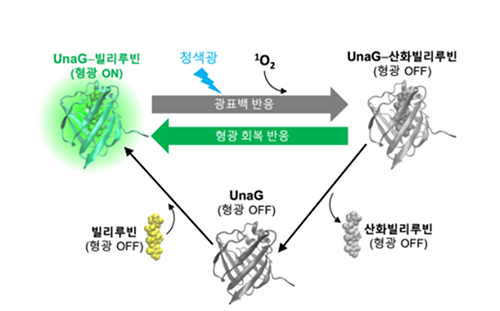
[Figure 2] Photobleaching-fluorescence recovery cycle of UnaG proteins
Injecting blue light into the UnaG protein results in a photobleaching reaction that stops fluorescence through photooxidation. Oxidized bilirubin is weakly bound to UnaG and escapes from the protein; the now empty UnaG binds to the new bilirubin molecule to restore fluorescence. This photobleaching-fluorescence recovery reaction is repeated as long as bilirubin exists in the solution.

[Figure 3] Super-resolution images of subcellular nanostructures obtained with UnaG fluorescent proteins
Super-resolution images obtained by labeling subcellular endoplasmic reticulum (left), vimentin filaments (center) and clathrin-coated pits (right) with UnaG fluorescent protein. On the left side of the dotted line in each image is the image of the normal diffraction limit resolution, and on the right side of the dotted line is the super-resolution image.
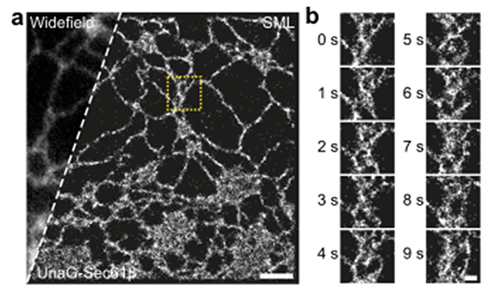
[Figure 4] Super-resolution microscopy of endoplasmic reticulum in live cells
(a) Normal resolution images (left of the dotted line) and super-resolution images (right of the dotted line) obtained by labeling living endoplasmic reticulum with UnaG fluorescent protein. (b) Video with super-resolution images obtained at one shot per second.

[Figure 5] Two-color live cell super-resolution microscopy
(a) The mitochondrial inner membrane (red) is labeled with a fluorescent substance called MitoTracker Red, and the mitochondrial matrix (green) is a two-color general resolution image (left) and a super-resolution image (right) obtained from living cells labeled with UnaG fluorescent protein. (b) General resolution images (left) and super-resolution images (right) of a single mitochondrion in the yellow dotted box portion of panel a. (c) The video start image (left) and the image after 5 min (right) in the blue dotted box in panel a. During the process of mitochondrial fission (yellow arrow) and fusion (white arrow), the matrix disappears and a narrow tube structure consisting only of the lipid membrane can be observed. (d) The video start image (left) and the image after 5 min (right) in the dashed white box on panel a. White arrows indicate the mitochondrial fusion process.



