Enabling Selective Degradation of Alpha-Synuclein Oligomers, the Main Cause of Parkinson’s Disease
A drug screening platform was developed to discover drugs that can selectively degrade α-synuclein oligomers.
The research results obtained by Professor Yoon Dae-sung’s group were published as a supplementary cover article in ACS Applied Materials & Interfaces.

▲ (From left) Dr. Lee Dong-tak of the School of Biomedical Engineering at KU (first co-author, currently a postdoctoral research at the Harvard Medical School), Jung Hyo-gi of the School of Biomedical Engineering at KU (first co-author,, integrated master-doctoral degree program), Dr. Park Dong-sung of the School of Biomedical Engineering at KU (first co-author), Professor Lee Jeong-hoon of the Department of Electrical Engineering at Kwangwoon University (corresponding author), Professor Lee Gyu-do of the Department of Biotechnology and Bioinformatics at KU, and Professor Yoon Dae-sung of the School of Biomedical Engineering at KU (corresponding author).
A research group led by Professor Yoon Dae-sung of the School of Biomedical Engineering at the College of Health Science developed a drug screening platform capable of discovering drugs that can selectively degrade α-synuclein (αS) oligomers, the main pathological driver of Parkinson’s disease, by coating αS oligomers on the surface of gold nanoparticles in the form of an amyloid corona.
* α-synuclein oligomers: Oligomers formed by the aggregation of α-synuclein protein molecules
(when the α-synuclein protein molecules present in the brain are aggregated and entangled with each other to form α-synuclein oligomers).
* Amyloid corona: A layered attachment of fiberized protein that aggregates to the surface of an object
(when aggregated and fiberized proteins are attached to the surface of an object, the proteins look like a layer formed on the surface of the object, which is referred to as an amyloid corona).
The results of the research were published online as a supplementary cover article in ACS Applied Materials & Interfaces (IF=10.4) on December 23, 2022.
* Title of article : Biomimetically engineered amyloid-shelled gold nanocomplexes for discovering α-synuclein oligomer-degrading drugs
As the average lifespan increases, South Korea is aging rapidly. A recent forecast predicts that, of a population of 47,740,000 in 2050, about 19 million people will be over the age of 65, accounting for 39.8% of the population, more than double the current number. The accelerated aging of the community is also increasing the percentage of patients with degenerative brain diseases. As of 2018, of the 7.5 million elderly population in Korea over the age of 65, about 750,000 people, corresponding to 10% of the elderly population, were diagnosed with a degenerative brain disease.
Lewy body dementia and Parkinson’s disease are types of degenerative brain diseases that have the highest rates of incidence after Alzheimer’s disease. Studies have shown that these degenerative brain diseases are caused by the misfolding of the αS proteins, and efforts are being made to develop a therapeutic agent targeted at the αS proteins. Recent studies have identified that αS oligomers formed by dopamine are the main pathological driver.
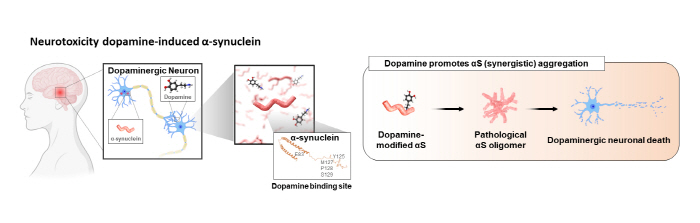
▲ Figure 1. A schematic diagram of the αS oligomers aggregated by dopamine, causing pathological symptoms in the brain of a patient with Parkinson’s disease.
However, due to the various degrees of aggregation, it is difficult to homogeneously purify the αS oligomers. Moreover, due to the absence of a fluorescent pigment targeted only at the oligomers, the drug discovery is facing challenges. In addition, large amounts of cost and time are required of the in vitro artificial protein synthesis and the related drug development because the αS proteins are expensive ($350/25ug) and a long time (at least one week) is needed to synthesize the αS aggregates.
In pursuit of a fast and homogeneous synthesis of the αS oligomers, the main cause of Parkinson’s disease, Professor Yoon’s group developed a method using gold nanoparticles and dopamine and employed it to develop a platform for rapidly screening drugs that can degrade αS oligomers. Gold nanoparticles, playing a catalytic role in the synthesis of the αS aggregates, were used to synthesize the αS aggregates at a concentration 100 times lower than the conventional protein synthesis concentration. In addition, proteins were deposited on the surface of the gold nanoparticles to form αS aggregates of a uniform size. Furthermore, amyloid-shelled gold nanocomplexes (ASGNs) were prepared by using dopamine in imitation of the form of the αS oligomers that cause pathological symptoms in the body.
When the prepared ASGNs are treated with a drug (enzyme or small molecule) targeted at the αS oligomers, the αS oligomers on the surface of the gold nanoparticles are degraded, exposing the surface of the gold nanoparticles and causing instability of the particles. This leads to the aggregation of the dispersed nanoparticles. Depending on the aggregation effect, a change of the optical properties of the gold nanoparticles occurs, thereby causing a red shift in the absorbance of the solution. The color change of the solution (red to purple) is observed to quantitatively evaluate the efficacy of the drug.
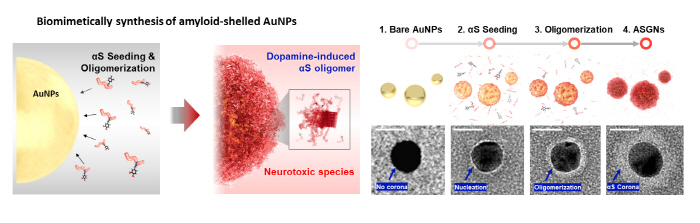
▲ Figure 2. A schematic diagram of the synthesis of ASGNs.
The research group validated the effectiveness of the developed drug screening platform by using protases that can degrade the αS oligomers and conducted the evaluation with 16 drugs that are related with brain disease treatments. Through this, the existing drugs that are known to have the capacity of degrading the αS oligomers were additionally validated, and the αS oligomer degradation capacity of the drug candidates were comparatively evaluated.
The conventional method for measuring drug efficacy based on fluorescence or protein shape requires tens of milligrams of proteins for protein aggregate synthesis and takes at least one week for the aggregate synthesis. Additionally, numerous pretreatment procedures are required for the fluorescent labeling. Contrarily, the αS oligomer degrading drug screening platform developed by the research group allows real-time rapid monitoring of the efficacy of thousands of drugs within several hours by using a single measurement instrument. Furthermore, the platform does not need a complicated pretreatment process and requires only several nanograms of the αS proteins for the measurement of the efficacy of one drug. This new drug screening method is much more efficient and cost-effective than the conventional method.
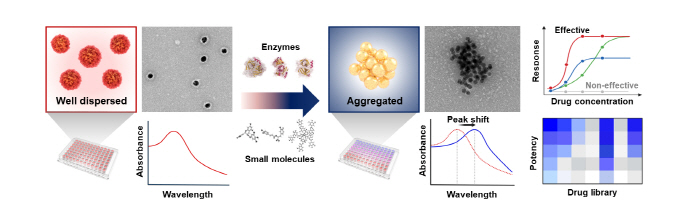
▲ Figure 3. A schematic diagram of the αS oligomer degrading drug screening performed by using ASGNs.
Through collaboration with ASTRION Inc., a biopharmaceutical company developing innovative drugs for intractable brain diseases, Professor Yoon’s group is using the drug screening platform to conduct massive drug screening with a library of the drug candidates against Parkinson’s disease. As a result, about 10 drug candidates have already been discovered for the degradation of the αS oligomers. These drug candidates have undergone additional validation regarding their capacity for degrading the αS aggregates, and based on the discovered drugs, a preclinical animal experiment study is being prepared to develop a therapeutic agent for Parkinson’s disease. In addition, Professor Yoon’s team will modify the proteins for coating the surface of the gold nanoparticles in the αS oligomer degrading drug screening platform to extend to various other drug screening platforms, including the τ-aggregate degrading drug screen and COVID-X therapeutic agent screening platforms.
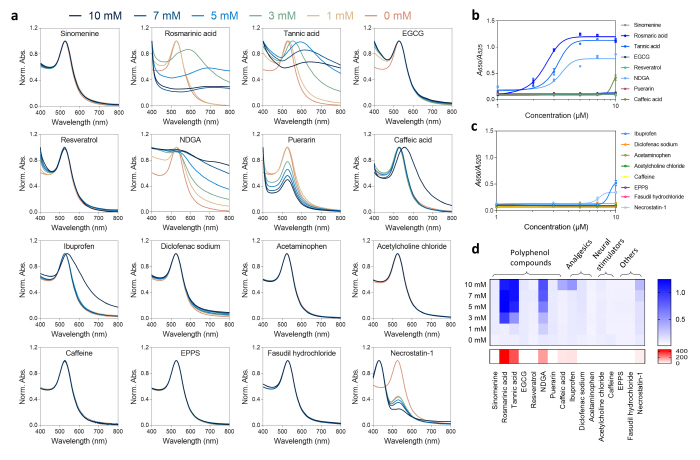
▲ Figure 4. Evaluation of the S oligomer degradation capacity of drugs performed by using the drug screening platform.



